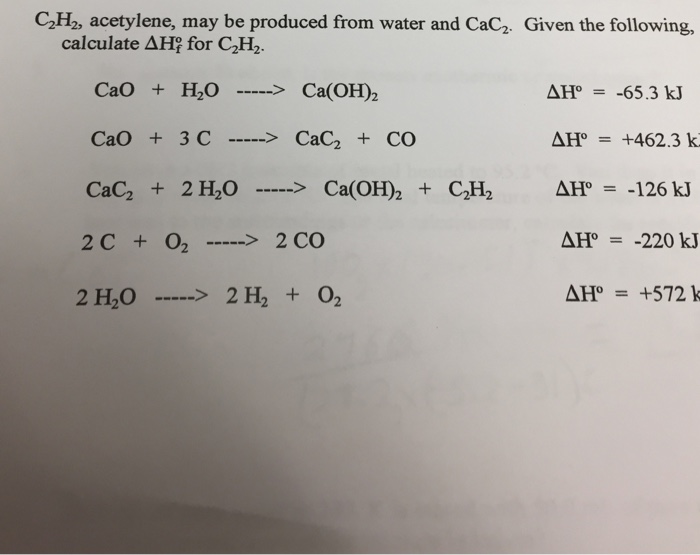Response FTIR spectra of CaO sample at (a) 700 °C, (b) 900 °C and (c)... | Download Scientific Diagram
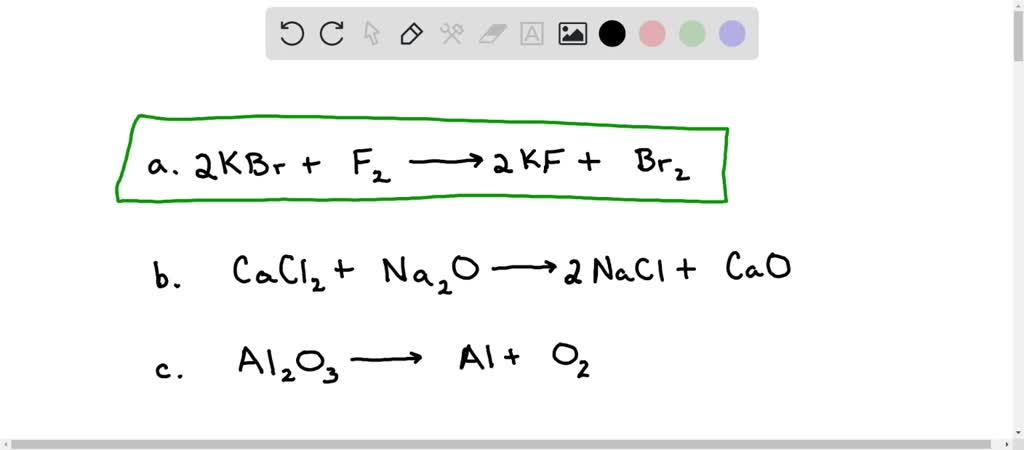
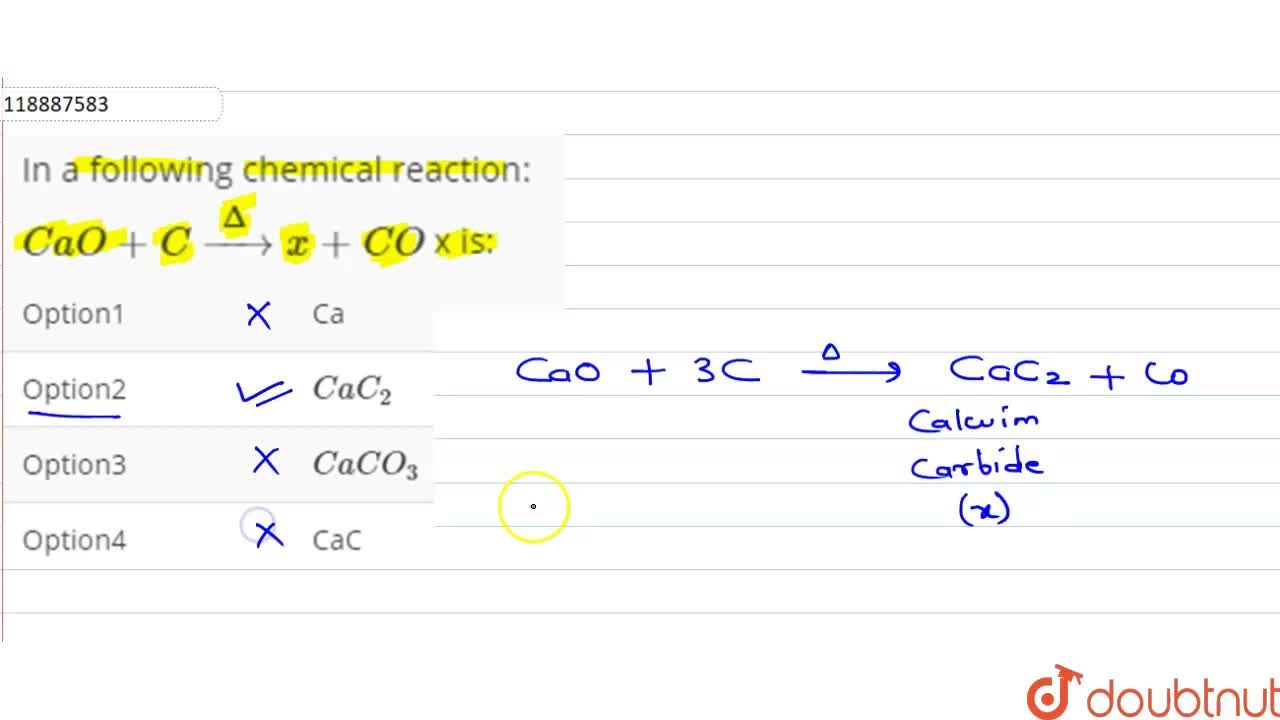
SOLVED: Balance the following reactions: a) KBr + F2 → KF + Br2 b) CaCl2 + Na2O → NaCl + CaO c) Al2O3 → Al + O2
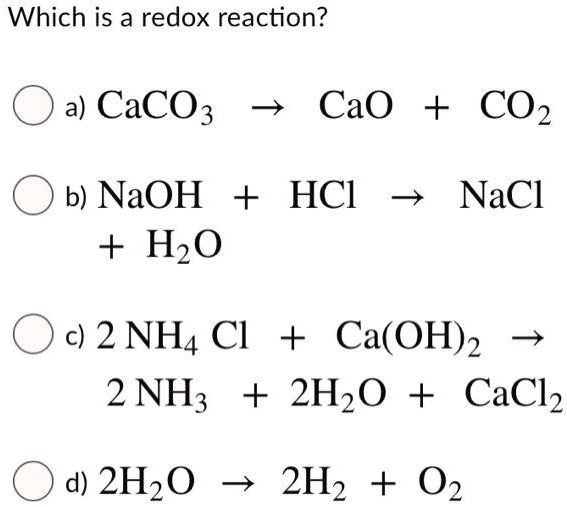
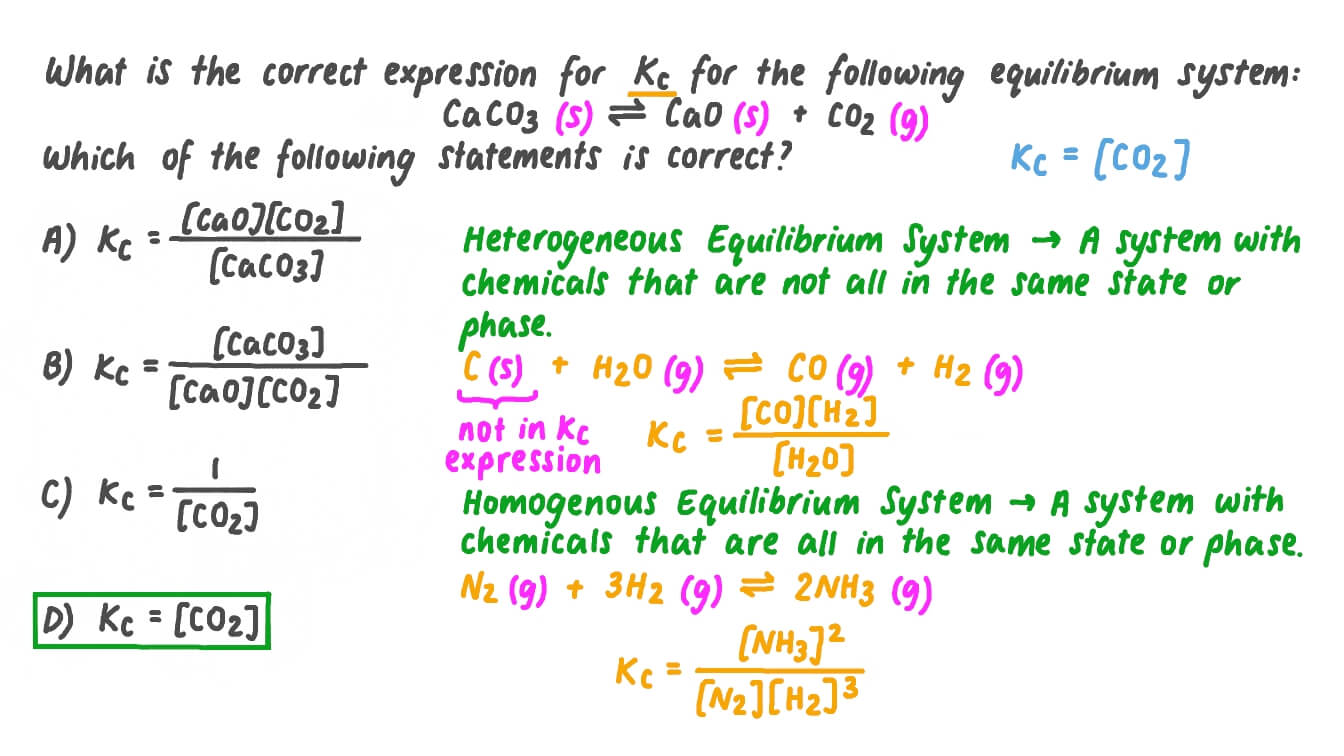
SOLVED: Which is a redox reaction? a) CaCO3 CaO + CO2 b) NaOH + HzO HCl NaCl c) 2 NH4 Cl + Ca(OH)2 2 NH; + 2Hz0 + CaClz d) 2Hz0 2H2 + 02

H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni-CaO-C - ScienceDirect

Question Video: Calculating the Mass of Calcium Carbonate Required to Produce a Given Mass of Calcium Oxide | Nagwa

i) CaO(s) + H2O (l) = Ca (OH)2(s) , Δ H180^oC = - 15.26 kcal (ii) H2O(l) = H2(g) + 12 O2 (g) , Δ H180^oC = 68.37 kcal (iii) Ca(s) +

Phase diagram of FexO-SiO2-CaO-MgO-"NiO"system, at PO2 = 10 −7 atm,... | Download Scientific Diagram

CO2 Adsorption on CaO(001): Temperature-Programmed Desorption and Infrared Study | The Journal of Physical Chemistry C

Chemical Equation A representation of a chemical reaction: C 2 H 5 OH + 3O 2 2CO 2 + 3H 2 O reactants products. - ppt download
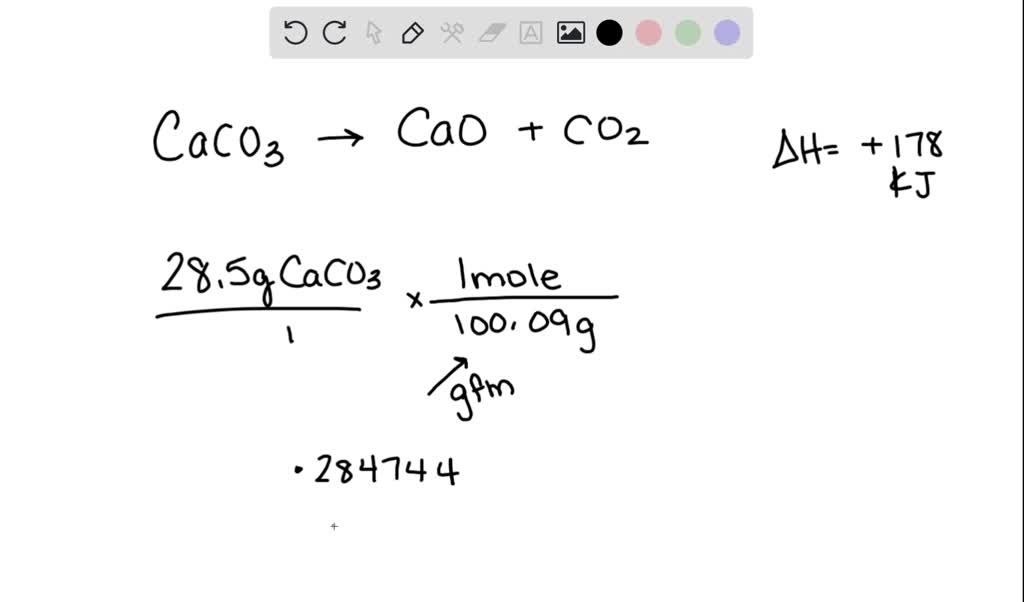
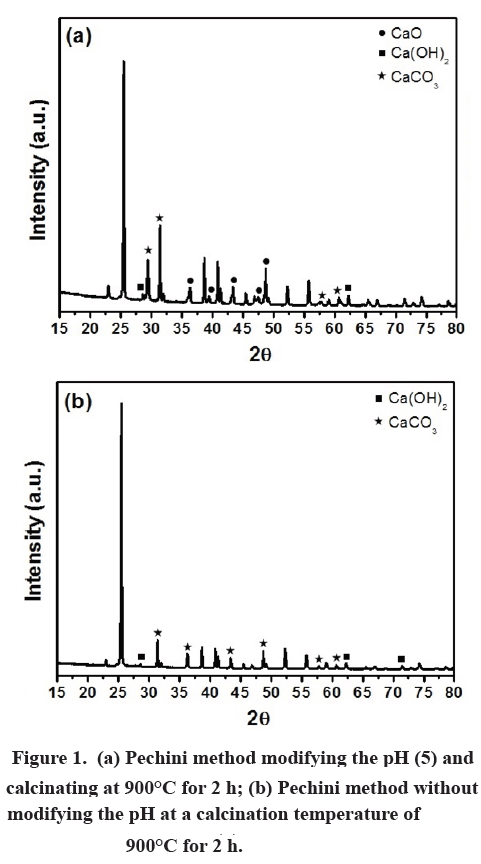
SOLVED: Consider the following thermochemical equation: CaCO3(s) CaO(s) + CO2 Ho = +178 kJ a) How many moles of CaCO3 are in 28.5g of CaCO3? moles b) How much heat must be

Insight into the role of CaO in coke-resistant over Ni-HMS catalysts for CO2 reforming of methane - ScienceDirect
amino acid (1mole)gas evolvedsalt(0.1999 kg) amino acid having i.2 NH2 gr. ii.1 COOH gr iii.2 COOH gr how to deal with such problem? iv.3 COOH gr

Chemical Reactions Follow the matter… 1. Chemical Reactions Two chemicals have interacted in some way so that a new substance or substances are formed. - ppt download