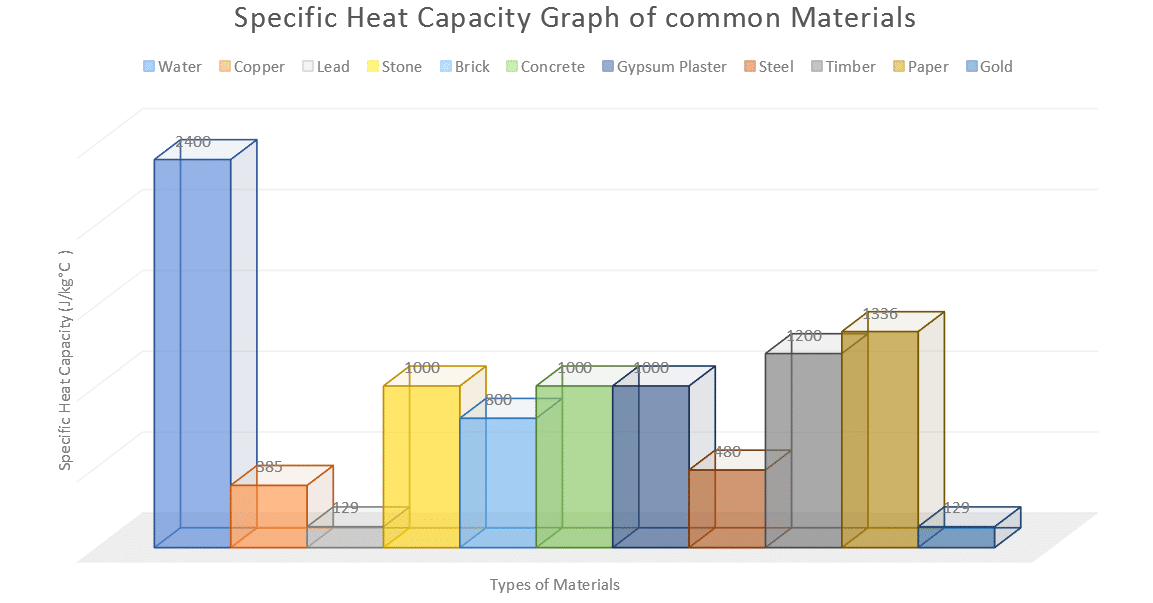
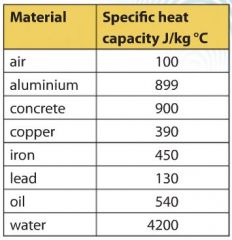
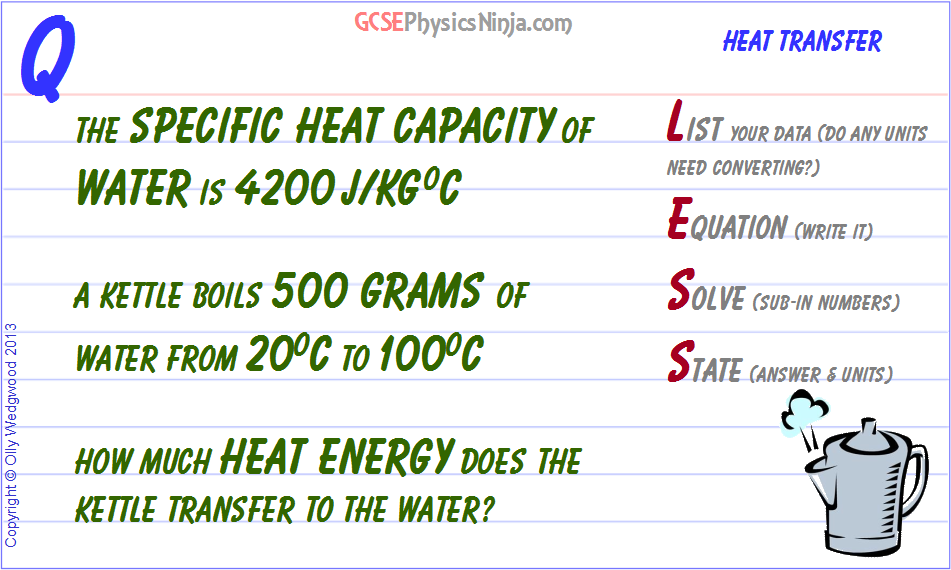
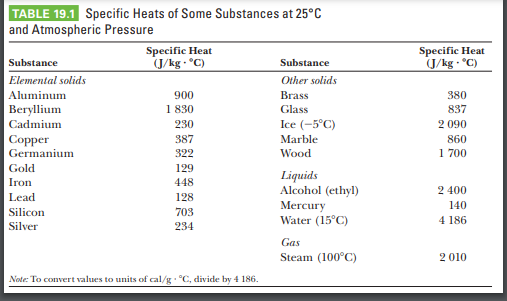
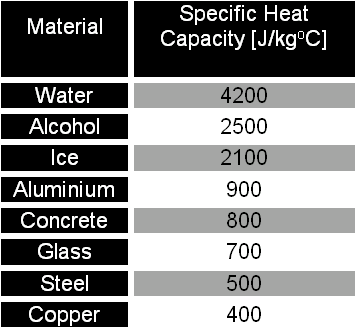
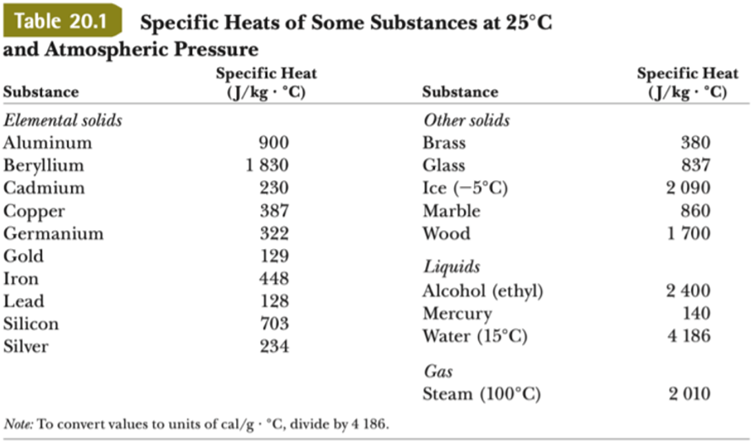
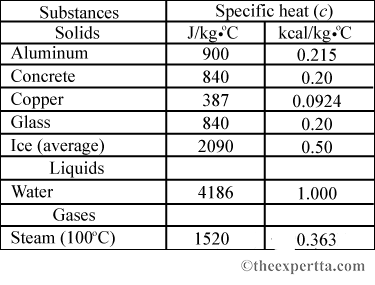
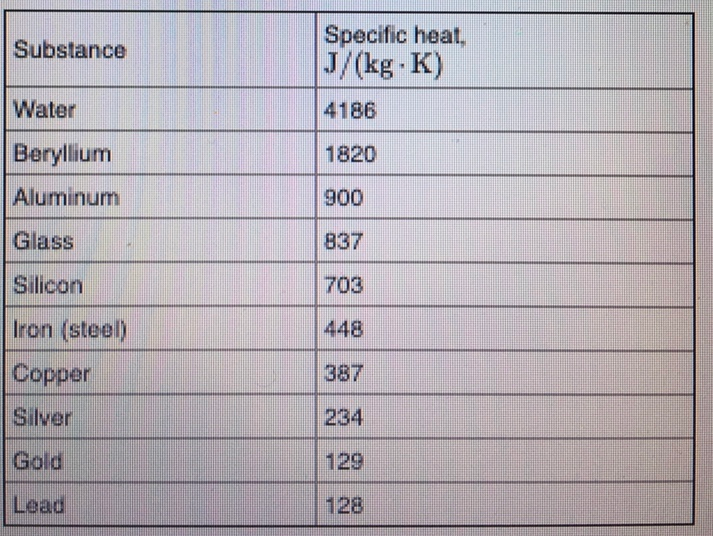
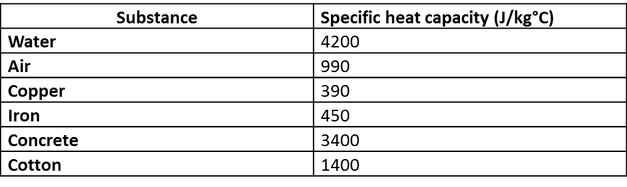
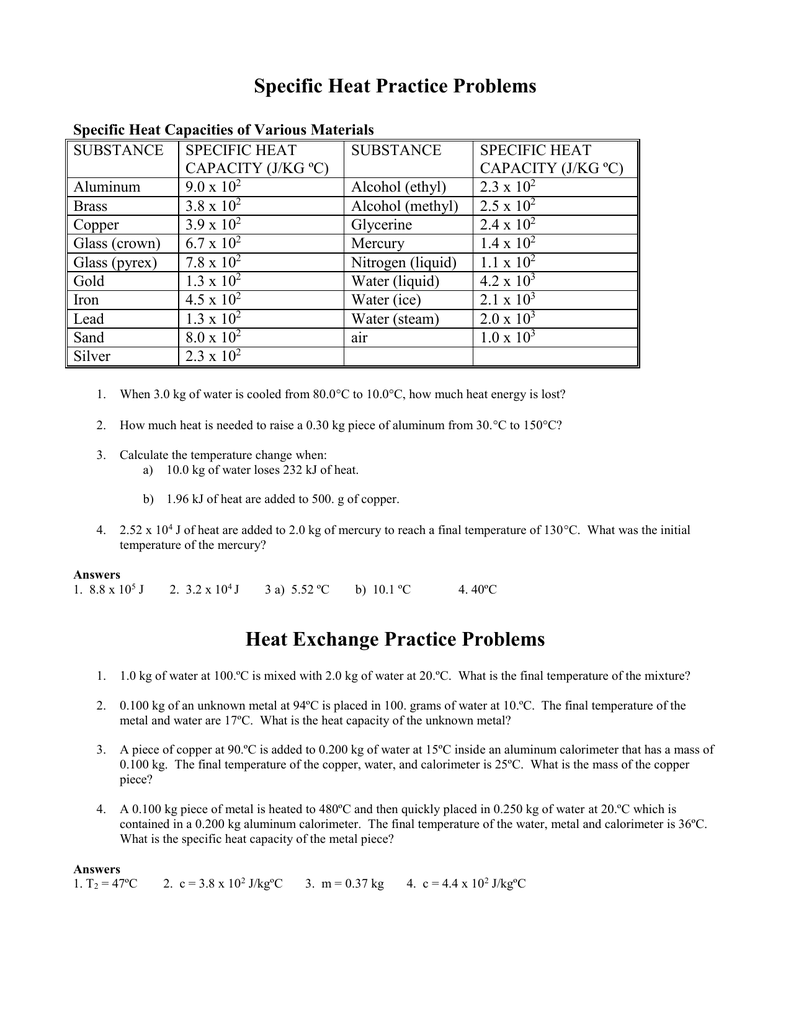
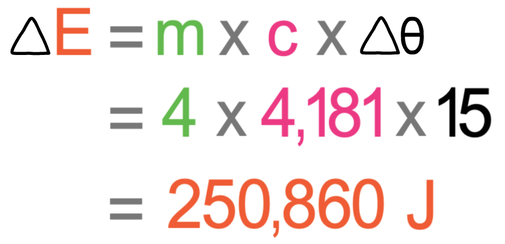SOLVED: Calculate the amount of heat needed to raise the temperature of 125g of water from 23*C to 100*C? Note that the specific heat capacity of water = J / kg: C 4186

Learning Outcomes: Rearranging equation for Specific Heat Capacity Topic Equation for Specific Heat Capacity Target Audience: G & T Teacher instructions. - ppt download

When you heat a substance, you are transferring energy into it by placing it in contact with surroundings that have a higher temperature. - ppt download
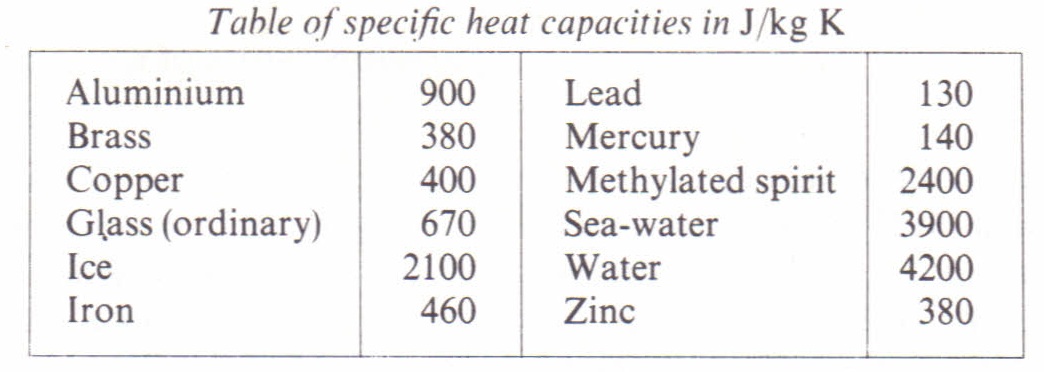
Specific heat capacity Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

The amount of heat energy required to convert 1 kg of ice at - 10^∘C to water at 100^∘C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity